When chlorine is introduced to a sodium bromide solution, whether as a gas or dissolved in water, chlorine replaces bromine through a displacement reaction. This occurs because chlorine is more reactive than bromine.
As a result of this reaction, the solution changes colour and turns brown due to the presence of the displaced bromine. The chlorine combines with sodium to form sodium chloride.
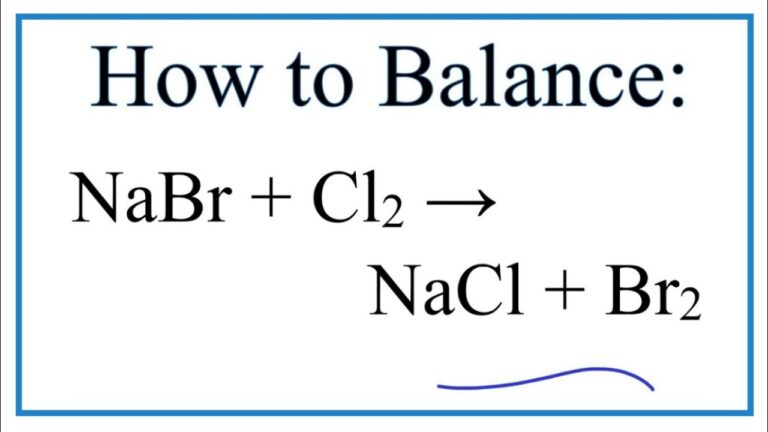
The chemical equation representing this reaction is as follows:
Chlorine + Sodium bromide → Sodium chloride + Bromine
Cl2(aq) + 2NaBr(aq) → 2NaCl(aq) + Br2(aq)
This kind of reaction, involving the swapping of elements between halogens, can be observed with all halogens.
Why Does Chlorine Displace Bromine From Sodium Bromide?

Basically, chemical reactions are fascinating processes that drive various natural and artificial transformations around us. Chemical reactions have always been at the forefront of scientific exploration.
Moreover, it offers us a glimpse into the intricate world of molecular transformations. Among these captivating reactions is the displacement of bromine by chlorine in sodium bromide.
This intriguing chemical phenomenon is driven by the unique reactivity of halogens, a group of elements that share common characteristics yet display distinctive behaviors.
One such intriguing reaction involves the displacement of bromine by chlorine in sodium bromide.
The Nature of Halogens
Halogens are a group of elements located in Group 17 of the periodic table. These elements include fluorine, chlorine, bromine, iodine, and astatine. Halogens are highly reactive due to their electron configuration
They only require one additional electron to achieve a stable electron configuration of eight valence electrons (except for astatine).
Reactivity Order of Halogens
Halogens exhibit a trend of reactivity, It is known as the “reactivity order.” Fluorine holds the highest reactivity among all halogens. It is followed by chlorine, bromine, and iodine. Astatine, being relatively rare and not well-studied.It is considered the least reactive halogen.
Displacement Reactions: Understanding the Chemistry
The displacement reaction between chlorine and sodium bromide is a classic example of halogen reactivity. When chlorine is introduced to a solution containing sodium bromide (NaBr). It initiates a reaction where chlorine replaces bromine to form sodium chloride (NaCl). This displacement can be represented by the chemical equation:
Cl2(aq) + 2NaBr(aq) → 2NaCl(aq) + Br2(aq)
The underlying reason behind this phenomenon lies in the reactivity order of halogens. Chlorine, being more reactive than bromine, is capable of dislodging bromine from its compound, sodium bromide, to form a new compound, sodium chloride.
Formation of Sodium Chloride and Bromine
As chlorine displaces bromine, sodium chloride (common salt) is formed in the solution. Simultaneously, the displaced bromine gives rise to a brown coloration in the solution.
It indicates the presence of free bromine. This brown colour serves as a visual cue for the occurrence of the displacement reaction.
Generalisation to Other Halogens
The chlorine and sodium bromide displacement reaction is not an isolated case. This type of reaction can be observed with all halogens, where a more reactive halogen displaces a less reactive one from its compound.
For instance, if iodine is introduced to a solution of sodium chloride, iodine will displace chlorine to form sodium iodide, and chlorine will be released.
A Glimpse into Halogens

Halogens, residing in Group 17 of the periodic table, possess unique characteristics that set them apart from other elements. These include fluorine, chlorine, bromine, iodine, and astatine.
They share a common desire for one additional electron to achieve a stable electron configuration, making them highly reactive elements.
The Reactivity Order of Halogens
One of the hallmarks of halogens is their reactivity order. Fluorine, the smallest and most electronegative of the group. It takes the crown as the most reactive halogen.
Following closely behind is chlorine, with its potent ability to engage in chemical transformations. Bromine secures the third position, while iodine demonstrates a more sluggish reactivity. Astatine, the least studied of the halogens, remains a tantalizing mystery in the reactivity spectrum.
FAQs
Why does chlorine displace bromine in terms of electrons?
Chlorine displaces bromine due to its higher reactivity in halogen displacement reactions. In terms of electrons, chlorine has a stronger ability to gain an electron and achieve a stable electron configuration with eight valence electrons.
This electron configuration is similar to that of noble gases.It makes it highly stable. Bromine, on the other hand, has seven valence electrons and seeks to gain one more electron to achieve the same stable configuration.
Chlorine’s higher reactivity allows it to outcompete bromine in acquiring an electron, leading to the displacement of bromine in various chemical reactions.
Why does chlorine displace bromine from potassium bromide?
When chlorine is introduced to potassium bromide (KBr) in a chemical reaction, chlorine’s higher reactivity allows it to displace bromine. Chlorine readily gains an electron from potassium, forming potassium chloride (KCl), and simultaneously displaces bromine, which then forms elemental bromine (Br2).
This displacement is a result of the reactivity order of halogens, where chlorine is more reactive than bromine.
Can chlorine displace bromine from bromides?
Yes, chlorine can displace bromine from various bromide compounds. This displacement occurs in halogen displacement reactions, where a more reactive halogen replaces a less reactive one in a compound.
For example, when chlorine is introduced to sodium bromide (NaBr), chlorine replaces bromine to form sodium chloride (NaCl) and liberates bromine gas (Br2).
Why can chlorine displace bromine from seawater?
Seawater contains a mixture of halide ions, including chloride ions (Cl-) and bromide ions (Br-). When chlorine is added to seawater.It can displace bromine from the bromide ions present in the water.
The displacement reaction occurs due to chlorine’s higher reactivity, enabling it to replace bromine in the seawater. This reaction is utilised in some water treatment processes and disinfection methods.
Why does chlorine diffuse faster than bromine?
The rate of diffusion of a substance is influenced by its molecular mass and temperature. Chlorine (Cl2) is a diatomic molecule with a lower molecular mass than bromine (Br2).
Due to its smaller size, chlorine molecules can move more rapidly and diffuse faster than bromine molecules. Additionally, at higher temperatures, the kinetic energy of molecules increases, leading to faster diffusion rates for both chlorine and bromine.
Can chlorine displace bromine from its solution?
Yes, chlorine can displace bromine from its solution in a halogen displacement reaction. When chlorine is added to a solution containing bromine, chlorine’s higher reactivity allows it to replace bromine in the solution.
The displaced bromine will be liberated from the solution, and the chlorine will form new compounds with the other elements present in the solution, depending on the specific chemical reaction involved.
Conclusion
The final section will provide thoughtful insights into the importance of chlorine displacing bromine from sodium bromide and its broader implications within the realm of chemistry.
Throughout this article, we have delved extensively into the fascinating domain of halogen chemistry, with a particular emphasis on the mechanism behind chlorine’s displacement of bromine from sodium bromide.
By gaining a deeper understanding of halogen reactivity and displacement reactions, we can truly admire the intricacy and sophistication of these chemical transformations.
This newfound knowledge holds immense practical significance, empowering us to regulate and foresee halogen reactions for enhanced safety and efficiency, whether in laboratories or industrial applications.